Research output per year
Research output per year
Dr
TW20 0EX
Molecular Genetics of Neural Tube Development
The neural tube is the embryonic precursor of the brain and spinal cord. Correct formation and patterning of the neural tube are essential for the development of a functional nervous system. Abnormalities in the early stages of development of the brain and spinal cord can have devastating consequences on the individual, in many cases leading to death at birth. Neural tube defects include spina bifida, an abnormality in the formation of the spinal cord, anencephaly, resulting from defects in formation of the brain, and craniorachischisis, which affects almost the entire brain and spinal cord. Neural tube defects affect around 1 in 1000 established pregnancies in the UK.
A major interest of the Neural Tube Development Group is centred on understanding the genetic causes of neural tube defects. Being able to understand the etiology of these defects from analysis of human patients is very difficult. We are using the mouse as a genetically accessible model organism in which to investigate the causes of neural tube defects. This tool allows us to perform detailed investigation into the genetic, cellular and molecular defects involved, and will allow us to test therapeutic agents that might prevent the occurrence of neural tube defects.
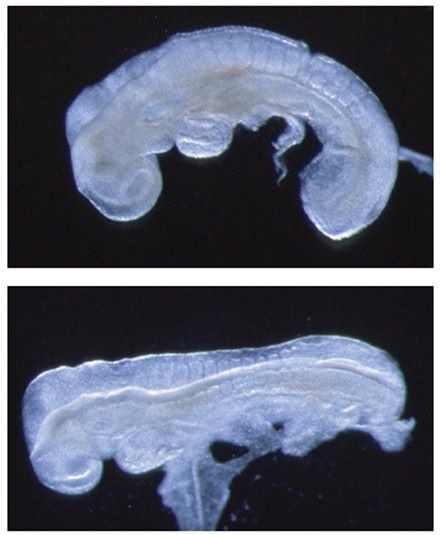
Figure 1. Crash and chuzhoi mutants with severe form of neural tube defect. Wild-type E8.5 embryo (upper panel) has initiated neural tube closure, but Crash mutant embryo (lower panel) has failed to initiate closure.
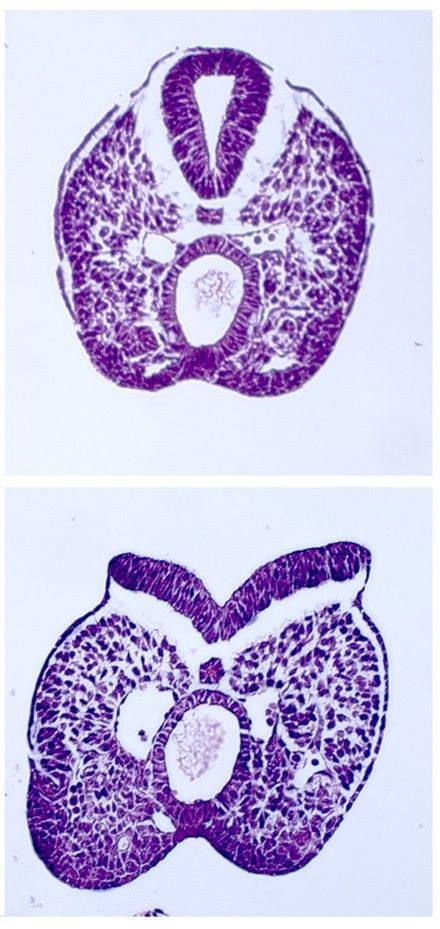
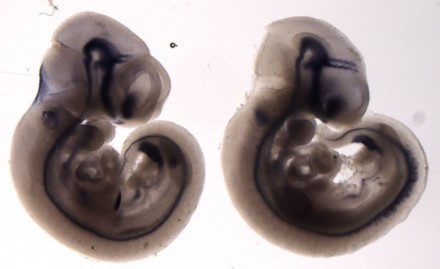
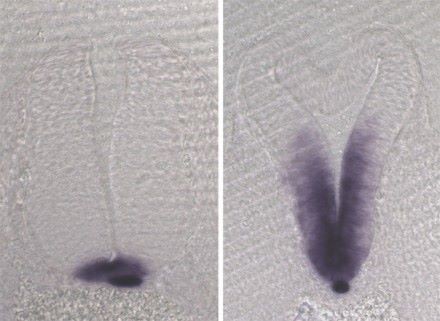
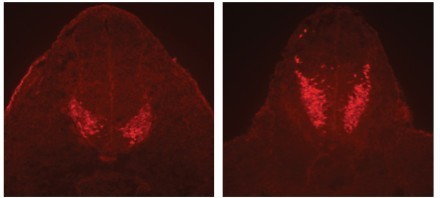
A significant focus of the group is on identifying the genes disrupted in new mutants that exhibit neural tube defects. These mutants can arise spontaneously, or can be generated through ENU mutagenesis, such as the on-going screens at MRC Harwell. Following identification of the mutation by positional cloning, we perform detailed molecular and cellular analyses to determine the role of the protein. Recent examples include two mutants that exhibit craniorachischisis, owing to failure to initiate neural tube closure (Fig 1). These mutants, Crash and chuzhoi, have disruptions in the genes Celsr1 and Ptk7. Both genes affect the planar cell polarity pathway. Interaction studies reveal a genetic interaction between these mutants, and we are interested in discovering how Ptk7 influences PCP. A third mutant identified recently is hitchhiker. This mutant exhibits spina bifida, exencephaly and polydactyly (Fig 2,3). We have shown that hitchhiker carries a mutation in Tulp3, with almost undetectable levels of protein. We are investigating the cellular causes of the neural tube defects while collaborations with other groups have been established to examine the defects in other organs including heart, lung and skin.
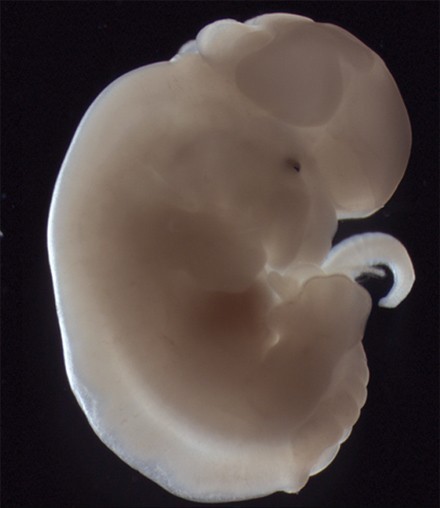
Figures 2 and 3. Homozygous hitchhiker embryo exhibits exencephaly and spina bifida
| Research group | |
|
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
1/06/11 → 31/05/12
Project: Research