Research output per year
Research output per year
Dr
TW20 0EX
Molecular Pharmacology of N-Methyl-D-aspartate glutamate receptors
Fast neuronal communication at synaptic contacts relies on neurotransmitter release from the presynaptic terminal and the majority of excitatory neurotransmission within the mammalian central nervous system is mediated by the neurotransmitter glutamate. Glutamate acts on a number of ligand gated ion channels often referred to as 'ionotropic' receptors which when opened permit ion flux across the cell membrane and result in a change in neuronal excitability. One subtype of ionotropic glutamate receptor is the N-methyl-D-aspartate receptor (NMDAR) and these receptors play a number of roles in normal and abnormal processes within the central nervous system (CNS), ranging from learning and memory to excitotoxic neuronal cell death following stroke or brain trauma. The involvement of NMDARs in such a broad range of CNS processes and disorders has triggered a great deal of interest in the molecular determinants of NMDAR function. This knowledge is not only required for teasing apart the complex mechanisms of receptor action but may also assist in the development of therapeutic strategies to combat the neurological damage induced by NMDAR overactivity.
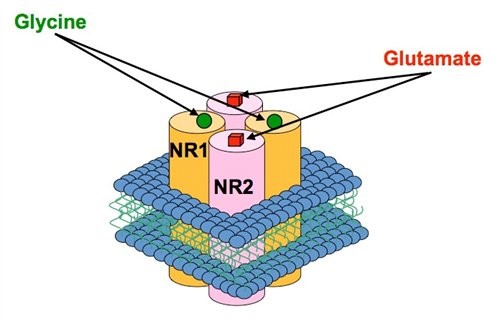
Figure 1. Each NMDAR ion channel is composed of two types of subunit, the NR1 and the NR2 (four subtypes exist NR2A-D) and these associate to form the receptor-channel complex. Both glutamate and the co-agonist, glycine are required to activate the receptor and binding sites for these agonists exist on the NR2 and NR1 subunits respectively. The incorporation of different NR2 subunits within the NMDAR can confer distinct pharmacological and functional characteristics.
My laboratory is interested in 1) understanding how specific regions or 'functional' domains within each type of NMDAR subunit can specify distinct pharmacological or functional properties. 2) how intracellular proteins associated with the NMDAR might influence pharmacological properties. 3) the impact of NMDARs with altered pharmacological properties or sensitivities on neuronal function. The laboratory is a using variety of molecular biological, biochemical and functional methods to understand the roles played by these domains in receptor function and pharmacology.
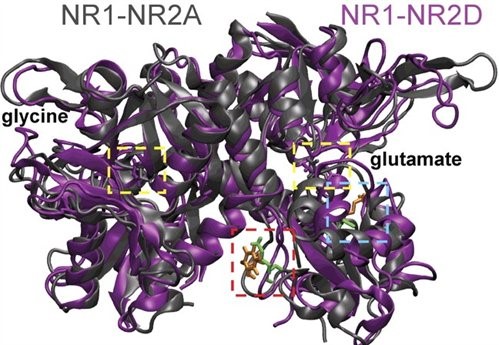
Figure 2. This is a homology model of the NR1 and NR2 glycine and glutamate binding pockets (agonists in yellow dashed boxes) that are thought to associate together within the receptor complex. Molecular dynamics simulations of the agonist binding pockets suggest differences in agonist inter-pocket residues (red and blue dashed boxes) between NR1-NR2A (grey) and NR1-NR2D (purple) subunit containing NMDARs (from Chen et al. 2008).
I am a Representative for The Physiological Society.
In 2015, UN member states agreed to 17 global Sustainable Development Goals (SDGs) to end poverty, protect the planet and ensure prosperity for all. This person’s work contributes towards the following SDG(s):
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Philip Chen (Speaker)
Activity: Talk or presentation › Invited talk