Research output per year
Research output per year
Dr
TW20 0EX
The role of centrosomes in early embryonic development in zebrafish
My interest lies in the roles of centrioles and associated proteins, as parts of the centrosome and cilium, in early vertebrate development, using zebrafish embryos and cell lines as model systems. The centrosome is the microtubule-organising centre of the cell. During cell division, the centrosome iforms part of the poles of the mitotic spindle that divides the duplicated chromosomes equally between daughter cells. The centrosome consists of two centrioles, cylindrical microtubule-based structures around which a complex of other proteins is formed (the pericentriolar matrix). The centriole is adapted in many cells to form the basal body from which cilia, hair-like, microtubule-based structures that protrude from the cell surface of many cell types.
Mutations in genes that encode components of the centrosome and cilium have been linked to a number of inherited, human developmental diseases: Bardet-Biedl, Alstrom, Joubert and oral-facial-digital syndromes - the 'ciliopathies'; a disease called primary microcephaly in which the size of the brain is reduced; and dwarfism.
My research is aimed at finding and characterising zebrafish embryos depleted of centrosome/centriolar/cilium proteins that give similar phenotypes in order to work out the developmental pathways in which these proteins and organelles are involved. I am particularly interested in proteins that contribute to the generation of cilia in early zebrafish embryos (red lines in Fig. 1A-C) . I use a variety of cell and molecular biology techniques in my research, including (confocal) (immuno)fluorscence microscopy and time-lapse imaging. I work with zebrafish embryos and zebrafish cell lines.
Recent projects have investigated how aggresomes (related to Lewy Bodies of Parkinson's Disease) affect centrosome and cilium function (Iqbal et al., 2020) and how centrosomes are affected in melanoma (Patel et al., 2020). We have also been investigating how zebrafish otolith formation is controlled by a polyketide synthase enzyme (Thiessen et al., 2019).
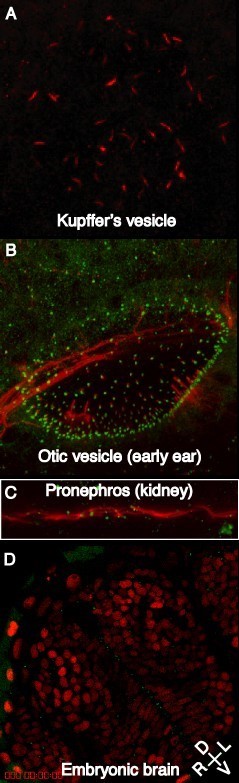
Figure. Centrosomes and cilia in zebrafish embryos. A) cilia (red) in Küpffer's vesicle (involved in determining left/right asymmetry); B) cilia (red) and basal bodies (green) in the otic vesicle (develops into the fish ear); C) cilia and basal bodies in the pronephros (embryonic kidney); D) centrosomes (green, GFP-centrin) line the apical surface of the neuroepithelium in the diencephalon of the developing 48 h embryonic brain, nuclei labeled red (H2B-RFP). Compass shows orientation: D = dorsal, V = ventral, L = left, R = right.
In 2015, UN member states agreed to 17 global Sustainable Development Goals (SDGs) to end poverty, protect the planet and ensure prosperity for all. This person’s work contributes towards the following SDG(s):
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
Research output: Contribution to journal › Article › peer-review
1/09/09 → 31/08/10
Project: Research